Home > About Hormona > Meet Ciara Horrigan, Regulatory Manager at Hormona


As you may have noticed, Team Hormona is expanding as we grow and introduce new features and exclusives. And as part of that expansion and those exclusives, so we’re so proud to announce Ciara Horrigan as the newest member of the team. Ciara is our new Regulatory Manager, helping us navigate the waters of medical regulation as we bring amazing new innovations.
Hey Hormona! My name is Ciara, pronounced Keer-rah, Horrigan. I live in County Tyrone, Northern Ireland, with my partner, pet rabbit, and cockapoo puppy.
My academic experience means I have a BSc in Biomedical Sciences, an MSc in Human Reproduction and Development, and a Postgraduate Certificate in Regulatory Affairs and Quality Compliance.
I was very fortunate to be able to study for my master’s part-time, while working full-time as an Immunoassay development and manufacturing scientist. And I chose that particular course as it dived deep into the Endocrine system, as well as the development of human embryos in the uterus. I learned so much from highly regarded professionals who have conducted research and published studies on many Endocrine-related disorders for both men and women.
My professional experience includes almost five years as a benchtop immunoassay IVD development scientist. However, most recently I worked as a Regulatory Consultant providing advice, particularly in the European Union and American landscape, in the area of medical device clinical research.

I suppose my interest began during my own experience of puberty as a teenager. Unfortunately, I always seemed to suffer a little more than my friends every month, and wondered why it worked so differently for everyone.
Science and biology were also my favorite subjects at school — I found learning about the human body and human development fascinating. So it was a natural progression of my interest in Science, and relating the changing female physiological processes to myself, which lead me to study for my master’s in Biomedical Science.
I’ve kept an eye on Femtech developments, as I always dreamt of steering my career in this direction. I came across a post on LinkedIn that listed “Five Femtech Companies To Look Out For,” and unsurprisingly, Hormona was mentioned!
So, I connected with Hormona co-founder Karolina and sent her a message expressing my interest in Hormona, and discussing any opportunities there may be with my skillset. Once I met Hormona’s COO, Jasmine, along with some of the other team members, I could feel their passion for Hormona’s mission, and I knew it was the place for me!
My responsibilities include ensuring our hormone tests are developed in compliance with medical device regulations. I’ll be working with our very talented software and scientific teams to ensure we continue to develop our products to the highest regulatory standard for our users. The sky’s the limit for where that takes us!

-


Dr Singh is the Medical Director of the Indiana Sleep Center. His research and clinical practice focuses on the myriad of sleep.

The importance of the follicular phase While it may not be the most fun part of the menstrual cycle, the follicular phase plays a key role in your reproductive health. As we mentioned, the follicular phase begins on the first

Understanding female hormones If you’ve never heard that women have specific feminine hormone levels, you’re probably wondering, “What are the female hormones and how many hormones do women have? Female hormones are hormones released in higher concentrations in a woman’s
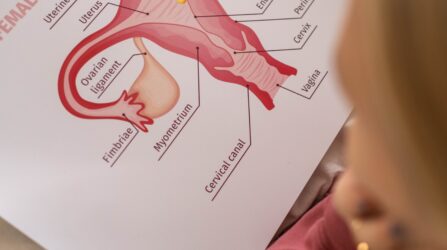
Understanding estrogen and its role in pregnancy Estrogen is one of the most important sex hormones, impacting every part of the fertility process. During your menstrual cycle, estrogen levels influence LH production, which triggers ovulation. While progesterone is responsible for
Hormona© 2025, All Rights Reserved
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
